In adult patients who had DKD* and T2D,
Patients were already taking a maximum tolerated dose of an ACEi or ARB.1
*With albuminuria >300 mg/day.
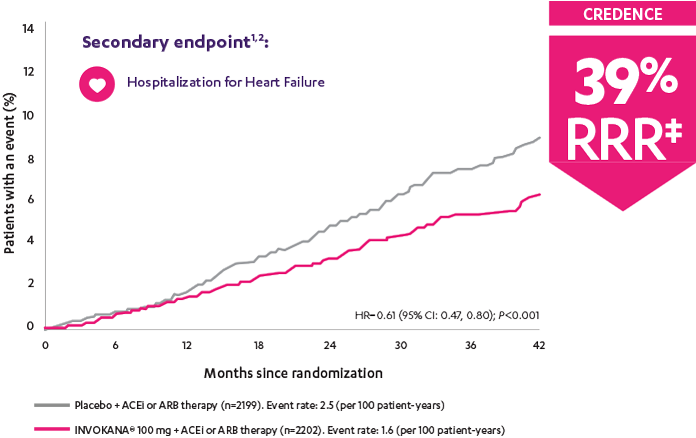
†Prespecified secondary endpoint.
‡RRR was calculated using the following formula: 100 × (1–HR).
ACEi=angiotensin-converting enzyme inhibitor; ARB=angiotensin receptor blocker; CREDENCE=Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation; DKD=diabetic kidney disease; HR=hazard ratio; RRR=relative risk reduction; T2D=type 2 diabetes.
Perkovic et al
CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) was a randomized, double-blind, placebo-controlled, parallel group, multicenter, event-driven clinical trial. The trial compared the effects of INVOKANA® 100 mg vs placebo in 4401 men and women with type 2 diabetes and diabetic kidney disease (described as chronic kidney disease with eGFR 30 to <90 mL/min/1.73 m2 and albuminuria [ratio of albumin to creatinine >300 to 5000 mg/g]) who were already taking a stable, maximum-tolerated, or labeled dose (for ≥4 weeks prior to randomization) of an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB). The mean eGFR of patients was 56.2 mL/min/1.73 m2 and the median urinary albumin-to-creatinine ratio was 927 mg/g. The primary efficacy outcome was the composite of end-stage kidney disease (dialysis, transplant, or eGFR <15 mL/min/1.73 m2), doubling of serum creatinine, or renal or cardiovascular (CV) death. Prespecified secondary outcomes included a composite of CV death or hospitalization for heart failure; a composite of heart attack, stroke, or CV death; hospitalization for heart failure; and a composite of end-stage kidney disease, doubling of the serum creatinine level, or renal death.3